Introduction
While comprehensive genomic analyses have identified genetic subgroups of diffuse large B cell lymphoma (DLBCL) when retrospectively performed on tumor biopsies (bx) from patients (pts) subsequently treated with R-CHOP (PMID 29713087, 29641966), it is unclear if these or similar assays can be applied in clinical practice. We aimed to determine if mutations (mut) detected in bx from newly-diagnosed DLBCL/high grade B cell lymphoma (HGBL) pts by clinical laboratory mutation analysis (CLMA) could predict for clinical outcomes following receipt of first-line therapy.
Methods
Pts with newly-diagnosed DLBCL/HGBL from January 2018-June 2022 whose bx underwent CLMA at the Penn Center for Personalized Diagnostics and received standard-dose R-CHOP or R-EPOCH as first-line therapy were included. Exclusion criteria were death due to non-lymphoma causes during first-line therapy and follow-up <1y after diagnosis if in remission. Pathologic workup of bx was performed as per institutional standard. Therapy was given at the discretion of the treating physician. Data were censored on July 1, 2023.
Results
One hundred thirty-seven pts met inclusion criteria, 15 were subsequently excluded and 117/122 pt bx underwent successful performance of CLMA (96%) with a median turnaround time of 17 days. Baseline characteristics, including mut occurring in ≥10% of cases tested, as well as genetic subgroup classification by LymphGen 2.0 based on available molecular data, are listed in the Table. R-CHOP was received by 72% of pts and R-EPOCH 38% of pts.
The overall response rate (ORR) was 84% (complete response rate [CRR] 69%), and with a median follow-up of 31.3 months, estimated (est) 2 year (y) progression free survival (PFS), est 2y overall survival (OS) and est median OS post-relapse were 70%, 83% and 21.4 months (mo), respectively. Univariate analysis (UVA) identified HGBL classification, MYC rearrangement (R) and TP53 mut as significantly predictive of progression at 2y; however, only TP53 mut remained predictive on multivariate analysis (MVA) (HR 2.3, 95% CI 1.1-4.8, P=0.03). Additionally, UVA identified International Prognostic Index (IPI) score ≥3, HGBL classification, MYC-R, MYC-BCL2 double hit lymphoma, TP53 mut and TNFRSF14 mut as significantly predictive of death at 2y; however, only HGBL classification (HR 5.0, 95% CI 1.1-22.7, P=0.04) and MYC-R (HR 4.4, 95% CI 1.0-18.0, P=0.04) remained predictive on MVA.
Given that TP53 mut predicted for both progression and death at 2y, we further analyzed bx with this mut. Forty-seven TP53 mut were detected in 42 pt bx, and were characterized as missense (mis) in 34, frameshift (fs) in 5, nonsense (non) in 5 splice site (ss) in 2 and other in 1. Thirty-eight (81%) TP53 mut were deemed loss of function (LOF), either as a mis/non mut designated as non-functional and/or LOF through The TP53 Database (https://tp53.isb-cgc.org/) or a fs/ss mut (PMID 22955915). Characteristics of pts with and without TP53 mut are listed in the Table.
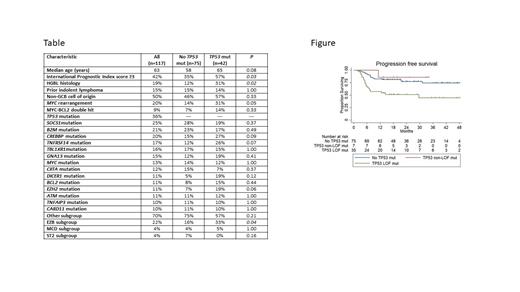
Significantly lower rates of ORR (71% vs 90%, P=0.009), CRR (55% vs 77%, P=0.01), est 2y PFS (57% vs 77%, P=0.006), est 2y OS (70% vs 91%, P=0.001) and est median OS post-relapse (6.1 mo vs not yet reached, P=0.001) were achieved by pts with vs without any TP53 mut. Est 2y PFS was 70% vs 33% ( P=0.01) and 2y OS was 85% vs 43% ( P=0.001) for pts with any TP53 mut treated with R-CHOP vs R-EPOCH. Additional analysis incorporating LOF status for TP53 mut revealed that for pts with at least one TP53 LOF mut vs only TP53 non-LOF mut vs no TP53 mut, 2y PFS was 51% vs 86% vs 77% ( P=0.002) as depicted in the Figure and 2y OS was 64% vs 100% vs 91% ( P=0.0004), respectively.
Finally, stratified log-rank demonstrated either inferior or a trend towards inferior 2y PFS for pts with vs without TP53 LOF mut harboring high-risk features, including IPI score ≥3 (42% vs 80%, P=0.009), HGBL histology (25% vs 70%, P=0.046), non-GCB cell of origin by Hans algorithm (52% vs 81%, P=0.01), MYC rearrangement (33% vs 48%, P=0.17), MYC-BCL2 double hit lymphoma (40% vs 67%, P=0.34) and EZB genetic subgroup (30% vs 81%, P=0.04).
Conclusions
TP53 mut identified through CLMA, specifically those resulting in LOF, predict for inferior clinical outcomes in pts with newly-diagnosed DLBCL/HGBL. Results of CLMA can be practically used to inform prognosis and/or identify pts for clinical trials investigating therapies targeting derangements caused by specificmut.
Disclosures
Landsburg:Curis: Research Funding; Epizyme: Membership on an entity's Board of Directors or advisory committees; Calithera: Membership on an entity's Board of Directors or advisory committees, Research Funding; ADCT: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Travel funding; Morphosys: Membership on an entity's Board of Directors or advisory committees. Nasta:Merck-Data Safety Monitoring Committee: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Research Funding; Genentech/Roche: Research Funding; Loxo/Lilly: Research Funding; Accrotech: Honoraria; Raphael: Research Funding; Ono Pharmaceutical: Research Funding; ADC Therapeutics: Honoraria; Millennium Takeda: Research Funding. Barta:Daiichi Sankyo: Consultancy; Acrotech: Consultancy; Affimed: Consultancy; Janssen: Consultancy. Schuster:Loxo: Consultancy; TG Therapeutics: Research Funding; Acerta: Consultancy; BiGene: Consultancy; Celgene: Consultancy, Research Funding; Nanovecter: Consultancy; Pharmacyclics: Consultancy; Merck: Research Funding; DTRM: Research Funding; Juno Therapeutics: Research Funding; Abbvie: Research Funding; Adaptive Biotechnologies: Research Funding; MustangBio: Consultancy; Incyte: Consultancy, Research Funding; Genentech/Roche: Consultancy, Research Funding; Janssen: Consultancy; Legend Biotech: Consultancy; Morphosys: Consultancy; Nordic: Consultancy; Regeneron: Consultancy; Novartis: Consultancy, Research Funding. Svoboda:Astra Zeneca: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Genmab: Consultancy; Atara: Consultancy; Adaptive: Consultancy, Research Funding; ADCT: Consultancy; Incyte: Consultancy, Research Funding; TG Therapeutics: Research Funding; SEAGEN: Consultancy, Research Funding; Merck: Research Funding; Pharmacyclics: Consultancy, Research Funding. Chong:MJH Healthcare Holdings, LLC: Honoraria; Genentech: Research Funding; Abbvie: Research Funding; Novartis: Honoraria; BMS: Honoraria; Beigene: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal